LENSai BioIntelligence Suite
Immunogenicity Screening
Boost your clinical strategy: in silico screening helps mitigate risks, enhance efficacy, and reduce time/costs
LENSai™ Integrated Intelligence Technology — an innovative platform leveraging advanced AI capabilities to deliver unparalleled protein analysis and lightning-fast immunogenicity screening. Powered by BioStrand’s proprietary HYFT® technology, LENSai Immunogenicity calculation combines HLA II binding and human proteome presence for comprehensive risk assessment.
Designed for clinical pathway success
High-throughput analysis
Built for high volume
Virtually limitless quantity can be screened, compared and ranked
Flexible implementation
Ability to integrate in your own pipelines and workflows
Optimize derisking
Pairing with humanization: designed to derisk and advance the best candidate
Key benefits
Avoid costly transgenics:
Satisfy requirements:
Versatile use:
Optimize workflows:
Optimal insights. Optimal candidates.
Maximize therapeutic benefits
Reference your antibodies against a database of all therapeutic antibodies
Data reference sources: Therapeutic structural Ab database VH/VL format (n≈2000), VHH library, bispecific library, and general proteins. Right figure: Parsed based on nomenclature (n ≈ 260)

Delivering multi-level analyses and comprehensive reporting
Immunogenic zones per sequence
Normalized score showing immunogenicity hotspots by combining HYFT Universal Fingerprint proteome screening and HLA II binding scores.
Dark Blue = 0 = low immunogenic potential
Dark Red = 100 = highest immunogenic potential
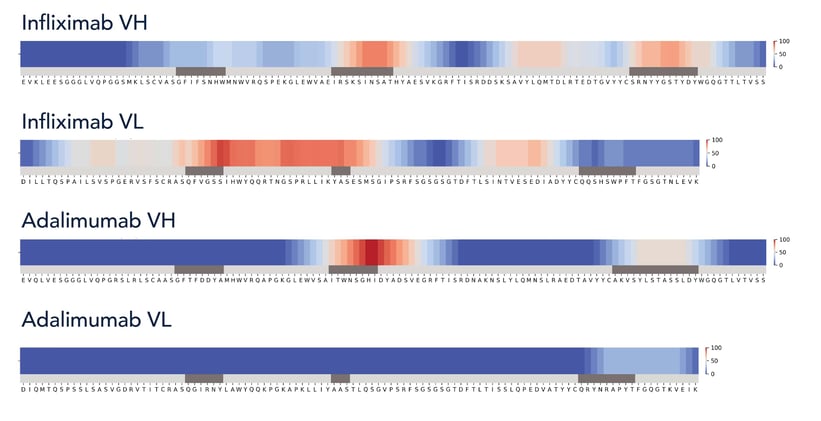
Get insights in potential immunogenicity risks introduced by engineering
Example bispecific formulation: BsAb VL1-VH1-VH2-VL2


